England to Offer Artificial Pancreas Technology to Tens of Thousands with Type 1 Diabetes
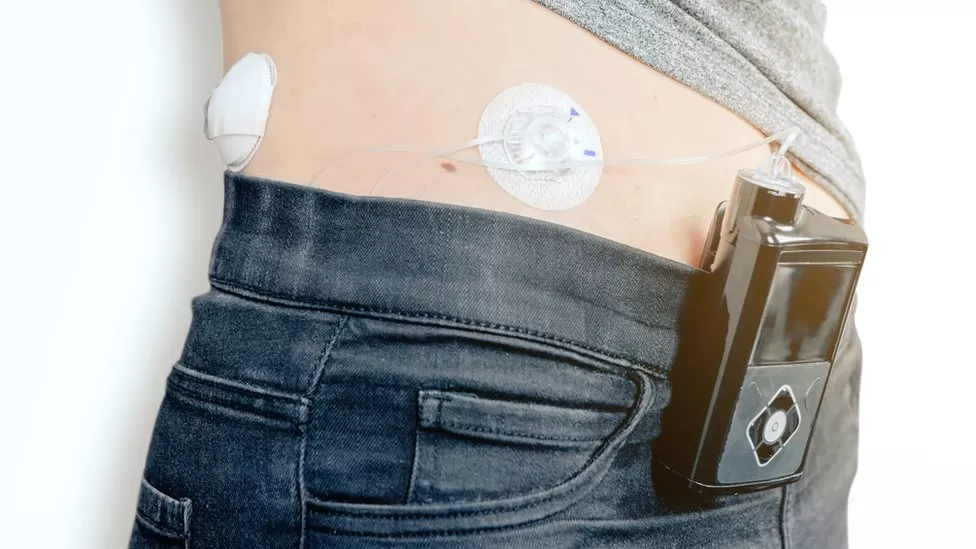
Tens of thousands of individuals living with type 1 diabetes in England are set to receive a groundbreaking technological aid known as an artificial pancreas. This innovative system utilizes a glucose sensor implanted under the skin to automatically calculate and administer insulin doses through a pump.
The National Health Service (NHS) will initiate contact with both adults and children eligible for this technology later this month. However, NHS officials caution that it may take up to five years for all eligible individuals to gain access to the device due to challenges in sourcing an adequate supply and the necessity of training additional staff in its usage.
Trials have demonstrated that this hybrid closed-loop system significantly enhances quality of life and reduces the risk of long-term health complications associated with diabetes. The National Institute of Health and Care Excellence (Nice) recommended its adoption by the NHS last year.
Approximately 300,000 people in the UK, including roughly 29,000 children, are affected by type 1 diabetes, necessitating meticulous monitoring of blood sugar levels and daily insulin administration. This new technology promises to automate these tasks, simulating the function of a healthy pancreas, albeit requiring mealtime input for accurate operation.
By preventing life-threatening fluctuations in blood glucose levels and improving overall blood sugar control, this technology aims to reduce the risk of complications such as heart disease, vision impairment, and kidney disease.
Scotland has also embraced this technology, with Wales and Northern Ireland potentially following suit. Gemma Lavery, a participant in an NHS pilot program, attests to the life-changing impact of the device, describing how it has alleviated stress and stabilized her diabetes management.
Prof Partha Kar, NHS national speciality advisor for diabetes, and Dr. Clare Hambling, NHS England diabetes clinical director, express optimism about the transformative potential of this technology for individuals with type 1 diabetes.
Diabetes UK chief executive Colette Marshall hails the roll-out of this technology as a significant milestone, echoing sentiments of excitement and anticipation within the diabetes community. Nice’s approval and the NHS’s five-year implementation plan mark a pivotal moment in the advancement of diabetes care in England.